The
Fukushima nuclear disaster caused radioactive substances to be spread
widely throughout the environment, and many foods were contaminated
with radioactivity. Currently, even though three years have passed
since the accident, the mechanisms of transference of radioactivity
from soil to plants are not yet well understood. This paper reports on
the state of research on radioactive contamination in agriculture, with
a focus on radioactive cesium. Note that radioactive iodine-131 has a
short half-life (8.02 days), so it is not being detected at this time,
and there are few reports of radioactive strontium.*
Tests for Radioactivity in Farm Produce
From
April 2012, the allowable concentration of radioactive cesium in foods
has been limited to 100 Bq/kg in general foods. The number of
investigations into concentrations of radioactive substances conducted
nationwide in Japan and the number of cases exceeding the limit are
shown in
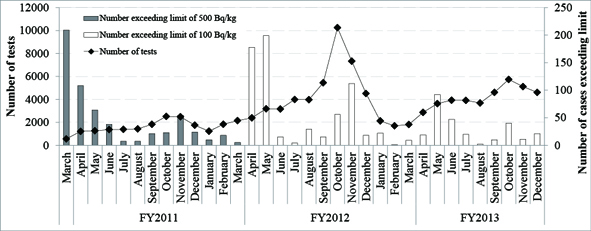
Graph 1.
The number of investigations and the number of cases exceeding the
limit fluctuate with the harvesting seasons, rising at times when foods
that absorb cesium easily, such as edible wild plants in the spring and
mushrooms in the fall, are being harvested. Foods exceeding the limit
in 2013 were mushrooms, edible wild plants, soy beans, bamboo shoots,
brown rice, buckwheat and komatsuna (Japanese Mustard Spinach).1
|
Graph 1. Number of Tests for Radioactive Substances in Foods (farm produce, vegetables)
A testing system
for rice was instituted in FY2012, when the limit was revised,
resulting in a big jump in testing in the fall. (Drawn by CNIC based on
results found on the Ministry of Health, Labour and Welfare website.)
|
Amounts Transferred from Soil to Plants
There
is a transfer coefficient that serves as an indicator of the amount
of radioactive substances in soil that will transfer to plants
(concentration of radioactive substance in plants ÷ concentration of
radioactive substance in soil). Generally, it is high for legumes and
root crops, but low for Curcurbitaceae and Brassicaceae.
In FY2011, Fukushima Prefecture found that vegetables with high
transfer coefficients when grown outdoors included edamame, with
0.0032 to 0.0040, and sweet potatoes, with 0.0049 to 0.0058. These
values indicate that if cultivated in soil with 1,000 Bq/kg, the
vegetables produced would contain 3 to 6 Bq/kg. Transfer
coefficients for these vegetables were measured again in FY2011 and in
FY2012, and the results were reported to have fallen to half what they
were.2
The transfere coefficient is thought to be predictable to some
degree, depending on the type of plant and soil properties, but there
have been cases where it was not.
Occurrence of High Concentrations of Radioactive Cesium in Rice and Investigation into the Causes
--Cannot be Explained by Transfer Coefficients Alone
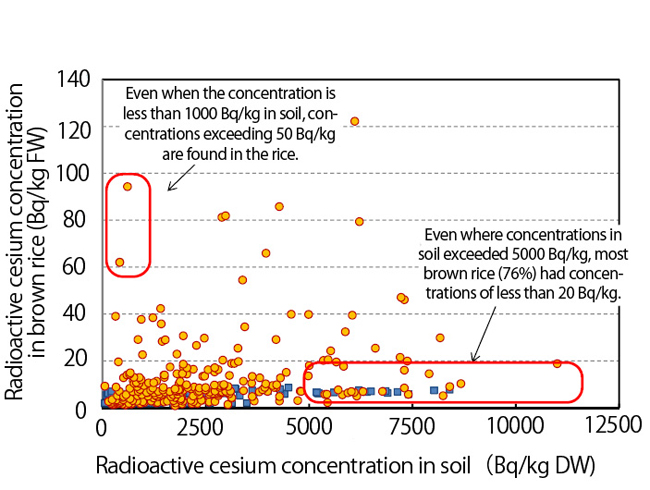
Graph 2 is
a plot of radioactive cesium concentration in brown
rice, grown in Fukushima Prefecture in FY2012, versus the concentration
in the soil in which it was grown. If in all cases the higher the
contamination of the soil, the more cesium was absorbed by brown rice,
all of the points on the graph should have been gathered in a line
sloping upward toward the right side, but that is not what happened.
Even when soil concentrations exceeded 5,000 Bq/kg, the cultivated
brown rice was found to contain only a small amount of contamination,
while even in fields with soil concentrations of less than 1,000 Bq/kg,
highly contaminated brown rice resulted.
|
Graph
2. Radioactive cesium concentration in soil versus its concentration in
brown rice (Ref. 3). (DW: dry weight, FW: fresh weight)
|
--Insufficient Potassium Causes Cesium to be Absorbed
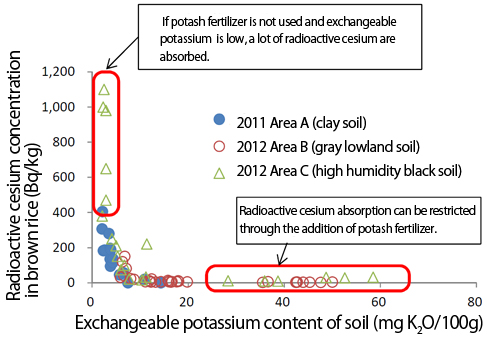
Cesium is chemically similar to potassium, so if a plant has insufficient potassium, it is as if it absorbs cesium by mistake.
Graph 3 shows
the relationship between soil potassium concentration and cesium
concentration in brown rice, based on a test cultivation of brown rice
in FY2012 in an area where brown rice cultivated in FY2011 had been
found to have radioactive cesium exceeding 500 Bq/kg. In light of this
relationship, it is clear that in soil with plenty of potassium
absorption of cesium is impeded, but in soil with insufficient
potassium, a high concentration of cesium develops in brown rice.3
|
Graph 3. Exchangeable potassium content of soil versus radioactive cesium concentration in brown rice (Ref. 3)
|
--Affected by Chemical Form of Cesium
Cesium ions are positively charged, so they are known to adhere
easily to the surfaces of minerals or humus from the decomposition of
organic matter that is negatively charged. The adhered cesium can be
displaced by ammonium or other positive ions, releasing it. Once
released, the cesium becomes water-soluble and dissolves. In addition,
cesium ions are similar in size to the gaps within molecules of clay
minerals, so cesium is known to enter these gaps and get “fixed” to the
clay minerals. Once fixed, it is very hard for the cesium to be
absorbed by plants.
An interesting experimental result was obtained in this regard. When
aquatic cultivation of rice was carried out with different
concentrations of radioactive cesium, even at a mere 0.1 Bq/liter in
water, the cultivated rice leaves had a radioactive cesium
concentration of 76 Bq/kg dry weight (
Fig. 1).
This suggests that the ease of transference to plants is strongly affected
by the amount of water-soluble cesium, but not by the total amount of
cesium contained in the soil.4

|
Fig. 1. Accumulation of radioactive cesium in rice plants grown in water culture medium with different concentrations (Ref. 4).
|
--The Paddy Environment and Rice Plant Characteristics
The farming area in Nihonmatsu City in which brown rice was found
to have contamination exceeding 500 Bq/kg in September 2011, consisted
of valley-bottom paddies surrounded by forest on three sides (with the
paddies distributed on terraces along the slopes). The water that the
paddies were drawing from the forest contains sufficient potassium and
magnesium to grow rice.
A survey
confirmed that the soil potassium concentration in the paddies was low,
and the clay content of the soil was small.
Although
the paddies were receiving water with abundant nutrients, for some
reason the soil did not contain sufficient potassium to prevent cesium
absorption. Even in areas where the soil of valley-bottom paddies
originally contained too few nutrients, there were agricultural
techniques that made rice cultivation possible using water from
forests. It is said that the valley-bottom paddy farmers of Nihonmatsu
City normally did not use very much potassium fertilizer.5,6 Therefore,
the soil had perhaps been chronically lacking in potassium.
The
rice plants themselves were discovered to have differing
characteristics. For the most part, the cesium concentration in rice
leaves was higher in the lower leaves and lower in the uppermost
leaves. In cases in which the brown rice had high concentrations of
cesium, however, the upper leaves of the plants had high
concentrations. The upper leaves grow during summer, so perhaps there
was a particular source of cesium for some reason in those paddies in
the summer. After that, the ears must have formed with high
concentrations of cesium.
The causes are currently being actively studied, with possibilities
noted such as hot temperatures in summer promoting decomposition of
organic matter, including fallen leaves containing radioactive cesium,
and water carrying that cesium in from surrounding areas.
Airborne Transport of Chemical Compounds of Radioactive Cesium
The
chemical form of the radioactive cesium that was carried by winds from
the Fukushima nuclear power plant is not well understood. Research has
been done analyzing fine atmospheric dust (aerosols) collected in
Tsukuba City after the accident. Some of the aerosol particles had
diameters of several micrometers, and others, 0.5 to 0.7 micrometers.
An elemental analysis detected cesium and sulfate ions together with
the latter, smaller aerosol particles. Perhaps the cesium was falling
to the ground as a sulfate salt.6 On the other hand, high
concentrations of radioactive cesium have been detected in spherically
shaped particles of several micrometers in diameter in some areas near
the Fukushima NPP.
After the accident, many kinds of vegetables were found with iodine,
cesium and other radioactive substances sticking to their surfaces and
their shipments were halted. Investigations on methods to remove the
radioactive substances from the surfaces revealed that about 60% could
be eliminated by washing with water, but that the addition of physical
stimuli such as ultrasonic cleaning did not change this ratio. The use
of acid, alkali or alcohol failed to produce a high removal ratio, but
the use of a reducing agent (1% sodium thiosulfate) used as an
antioxidant for foods increased the elimination of iodine. This was
observed to be due to conversion of the hard-to-dissolve iodine
molecules (I
2) to water-soluble iodine ions (I
-).7
Investigation of Circular Agriculture
As
an example of circular agriculture, hay and other feed is grown in
soil; livestock eat that and produce manure; the manure, together with
plant and other waste, is used to produce compost; and the compost is
added to the soil to provide nutrients for plants, as illustrated by
the relationships in
Fig. 2.
After the nuclear accident, the allowable amount of radioactive cesium
in fertilizers was limited to 400 Bq/kg, and in feed for cattle, hogs
and other livestock, to 300 Bq/kg, and in some cases, the circular
relationship was broken.
In FY2011, the muscles of goats continuously given feed with 3,900
Bq/kg were found to contain cesium of 130 Bq/kg, and their manure
contained 150 Bq/kg, resulting in compost containing 890 Bq/kg.
Research findings on plant cultivation using contaminated compost show
that cultivation of vegetables solely with compost of about 800 Bq/kg
produce eggplant, maize, soybeans and ginger with less radioactive
cesium than the detection limit of 20 Bq/kg. In order to produce the
highest degree of contamination, the compost was not mixed with soil,
but used alone for cultivation.8 It bears noting that if the compost
used is not fully matured, it may contain ammonium ions, which displaces and
frees cesium adhering to the soil, possibly increasing the amount of
cesium that can be taken up easily by plants, so caution is necessary
if using compost that is not fully matured.

|
Fig. 2. Conceptual diagram of sustainable agriculture (example).
|
Efforts to Curb Exposure During Farm Work
If
tilling is not carried out, almost all of the radioactive cesium is
fixed within the top 5 cm from the soil surface.6 In this case, if the
surface layer of the soil is removed, the air dose rate decreases, but
this requires removal of fertile soil, and it also creates problems on
where to put the discarded radioactive waste.
In Fukushima Prefecture, efforts have been made to decrease the amount
of radioactive cesium in the surface layer by deep tilling of the soil
or by interchanging the upper and lower soil layers. When “plow
tilling” was tried, in which the radioactive cesium in the surface
layer was plowed into the lower layer, the radiation levels in flooded
paddies were reduced by about 50%, and the greatest effects were
achieved in fields, where reductions of nearly 90% were reported (
Table 1).
| Test site |
Plow type |
Tilling
Depth
|
Prior
use of rotary till |
Air dose rate |
| Before
plowing |
After
plowing and rolling compaction |
Rate
of decrease |
| (cm) |
(yes/no) |
(μSv/h) |
(μSv/h) |
(%) |
| Flooded
paddies |
Iitate Village |
Plow with jointer |
30 |
No |
1.63 |
0.52 |
68 |
| Iwaki City |
Plow with jointer |
30 |
No |
0.41 |
0.2 |
51 |
| Kori Town |
Plow with jointer |
30 |
Yes |
0.69 |
0.41 |
41 |
| Motomiya City |
Two-step tiller paddy
plow |
30 |
Yes |
1.02 |
0.45 |
56 |
| Fields |
Minami Soma City |
Two-step tiller field
plow |
45 |
No |
2.13 |
0.41 |
81 |
| Tamura City |
Two-step tiller field
plow |
45 |
No |
1.3 |
0.17 |
87 |
| Fukushima City |
Two-step tiller paddy
plow |
30 |
No |
0.46 |
0.15 |
67 |
| Nihonmatsu City |
Two-step tiller paddy
plow |
30 |
Yes |
0.65 |
0.34 |
48 |
|
Table 1. Plow tilling air dose rates and their reduction (at 100 cm above ground surface).(Ref. 2)
|
The
natural world is subject to complex influences. Observing nature in
detail, following up on slight hints, setting up experiments, and
elucidating the mechanisms of radioactive contamination require serious
work. In practical field tests, long periods of time are needed between
planting seeds and harvesting crops, and in the case of rice, no more
than one verification can be accomplished each year. It is thought that
a certain amount of time will be needed in order to ascertain the
mechanisms of the transference of radioactive substances into food
crops. Also, reducing exposures during farm work is considered an issue
of importance to the continuation of agriculture.
(Nobuko Tanimura, CNIC)
[note]
*When distributed
foods were investigated in Fukushima, Iwate, Tochigi, Niigata, Ibaraki,
Kanagawa, Saitama and Kochi prefectures, radioactive strontium was
detected in seven of the 20 foods tested. The concentrations ranged
from 0.016 to 0.039 becquerels per kilogram (Bq/kg), which amounted to
about 1% of the radioactive cesium concentration.
“Results of testing
for radioactive strontium and plutonium in foods (results from Feb-May,
2012)” found on the Japan Ministry of Health, Labour and Welfare
website (http://www.mhlw.go.jp/stf/houdou/0000028846.html)-- in Japanese
[References]
(1) “Coping with
radioactive substances in food” (Shokuhinchu no hoshaseibusshitsu e no
taio). Japanese Ministry of Health, Labour and Welfare website.
http://www.mhlw.go.jp/shinsai_jouhou/shokuhin.html
(2) “Regarding research
on radioactive substances in the field of agriculture” (Nogyobunya ni
okeru hoshaseibusshitsu shiken kenkyu ni tsuite). Fukushima
Agricultural Technology Centre.
http://www4.pref.fukushima.jp/nougyou-centre/kenkyuseika/kenkyu_seika_radiologic.html
(3) “Regarding causes of
and countermeasures to high concentrations of radioactive cesium in
rice” (Hoshasei seshium nado no takai kome ga hassei suru yoin to sono
taisaku ni tsuite). Japanese Ministry of Agriculture, Forestry and
Fisheries. http://www.maff.go.jp/j/kanbo/joho/saigai/s_seisan_1.html
(4) Nemoto Keisuke. “New knowledge on absorption by crops” (Sakumotsu no shinchiken). Gakujutsu no Doko, Oct. 2012, pp.22-26.
(5) “Results of an
investigation of paddies in Obama-cho, Nihonmatsu City, on Oct. 17,
2011” (Nihonmatsu-shi Obama-cho no suiden ni okeru chosa kekka Heisei
23 nen 10 gatsu 17 nichi). Agriculture, Forestry and Fisheries
Industries of Fukushima Prefecture.
http://www.pref.fukushima.jp/keieishien/kenkyuukaihatu/gijyutsufukyuu/05gensiryoku/231017_obama.pdf
(6) Nakanishi Yuko
(2013), Dojo Osen Fukushima no Hoshaseibusshitsu no Yukue (Soil
Contamination: The whereabouts of Fukushima’s radioactive substances),
NHK Books, 220 pp.
(7) Vegetable Analysis
Group, Ad Hoc Committee on Safety Measures for Radioactive Iodine and
Cesium, Japanese Society of Radiation Safety Management. “Interim
report on methods of removing radioactive substances adhering to
vegetables contaminated by fallout from the Fukushima Daiichi nuclear
accident” (Fukushima Daiichi genpatsujiko ni yotte osen sareta yasai ni
fuchaku shita hoshaseibusshitsu no jokyoho ni kansuru chukan hokoku).
Isotope News. 2011, No. 689, pp. 55-58.
(8) Report by Manabe
Noboru, et al., at a study briefing session on the effects of
radioactivity on agriculture, livestock and marine products, The
University of Tokyo School of Agriculture and Life Sciences.
Return to
Radiaion Exposure page
Return to NIT 159 contents

